Many of us may hear about UV-A, UV-B and UV-C ultraviolet radiation. However, not all of us know what’s the difference between them. In order to explain it better, we will firstly introduce the concept of solar radiation.
Solar radiation
Solar radiation, also called solar source, is the energy emitted by the sun, which could travel through space by electromagnetic waves in all directions.
Spectral Distribution of Solar Radiation
Solar radiation reaches the earth’s surface in the form of electromagnetic radiation or light.
The wavelength (λ) and the frequency (µ) of the electromagnetic waves obey this equation: C = µ * λ (in which µ is the frequency of the light). These two factors are considered incredibly important in determining the energy, the visibility and the penetration power of solar radiation.
The wavelength is so short that it is expressed in nanometers (nm). Energy of different fraction of radiation is called photon, which is inversely proportional to its wavelength.
The sun emits short wavelength energy, mainly in the form of ultraviolet radiation, which is characterised by its visible and near infrared band, with a wavelength of between 0.2 and 3 µm (200nm-3000nm). Around 99% of the short-wave solar radiation that reaches the earth surface is between 0.2 and 3 µm in wavelength while the majority of the terrestrial wave radiation can reach up to 3.5 and 50 µm.
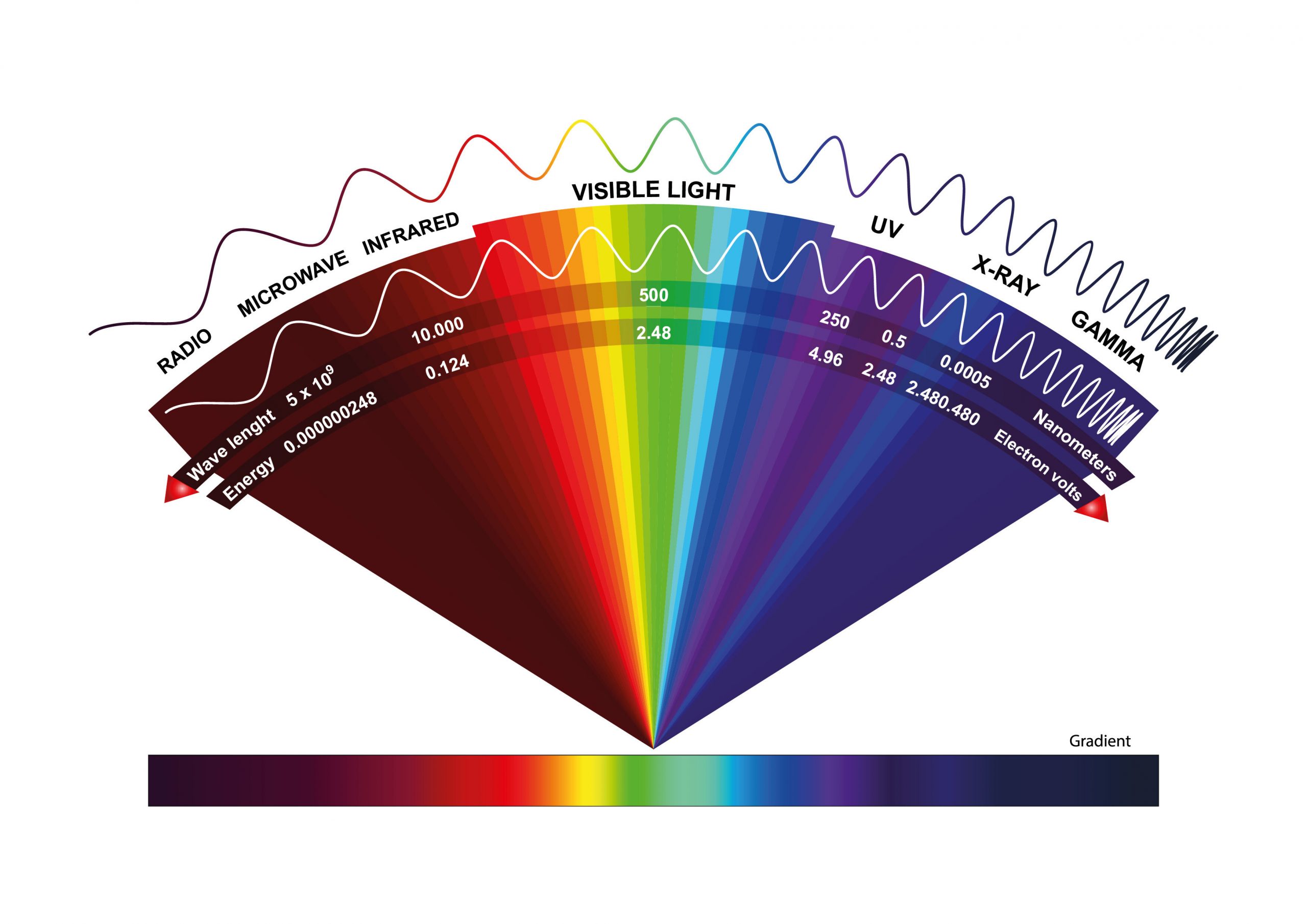
- The visible light (Between 400nm <λ <700nm) is a radiation type that can be precepted by human eyes. It includes the following colors:
Violet (0.42 µm or 420nm)
Blue (0.48 µm)
Green (0.52 µm)
Yellow (0.57 µm)
Orange (0.6 µm)
Red (0.7 µm)
Violet light is more energetic than red light as it has smaller λ figure.
Radiation with shorter wavelengths than that of violet light is called ultraviolet radiation.
Since the speed of light (C) is a constant, colors change because they have different frequency and wavelength.
If two rays of light have the same frequency, their wavelength will keep the same and they will present the same color.
C = µ * λ.
C: If µ is the same for each ray of light, it implies the λ is the same, then the color also will be the same.
- The ultraviolet light is between 100 and 400nm.
- The near-infrared light is between 700 and 4000nm
Waves in the range of 0.25 µm to 4 µm are called the short-wave spectrum.
Ultraviolet Radiation
Johannes Ritter discovered the sun, apart from emitting visible light, would also emit invisible radiation, with a wavelength shorter than violet light. This band is called ultraviolet.
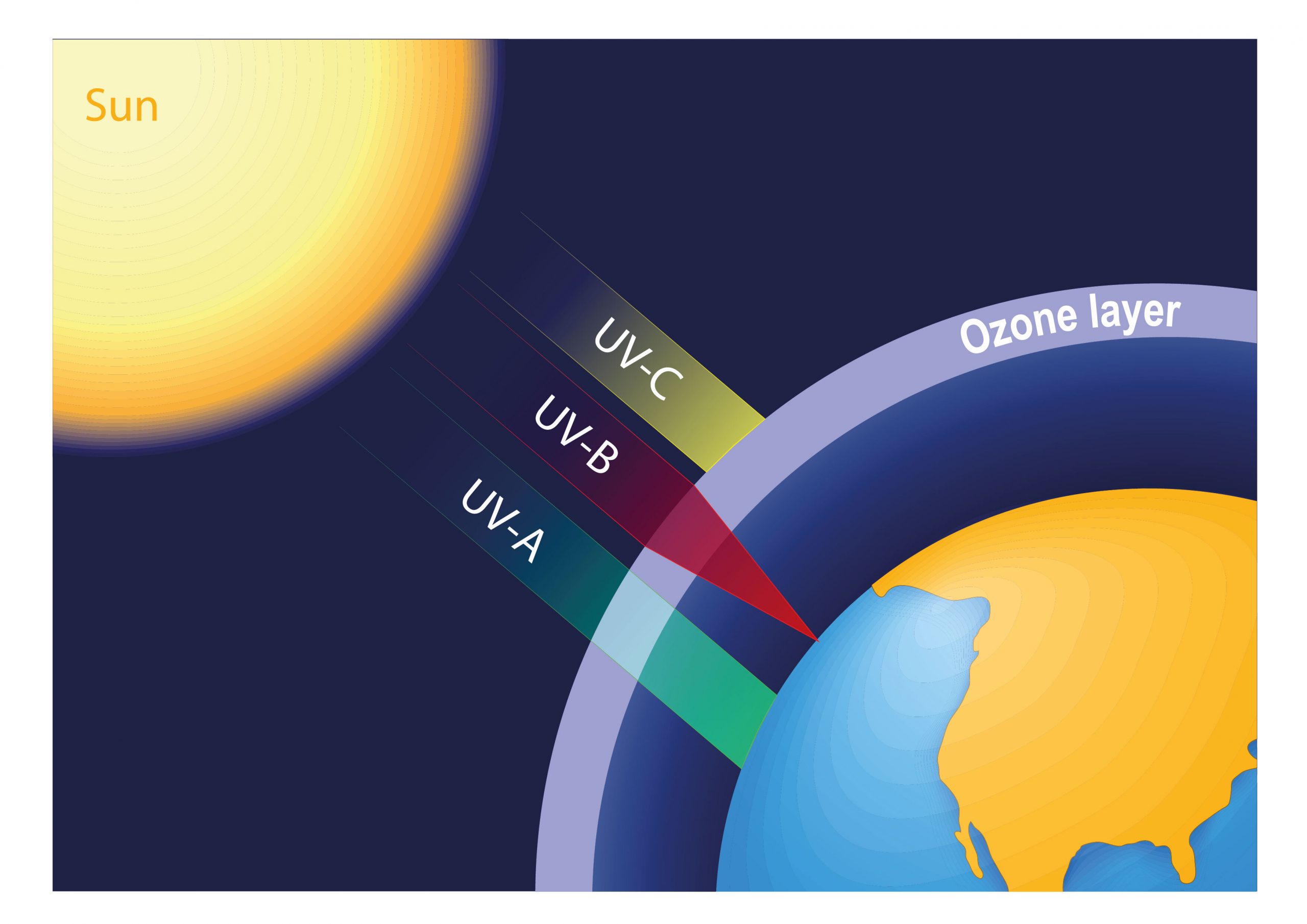
Among the large amount of energy emitted by sun to the earth, only 6% to 7% is correspondent to the ultraviolet (UV) light. This ultraviolet radiation is an invisible radiant energy that covers a wavelength range between 100nm and 400nm.
Usually the ultraviolet (UV) light is classified into three categories (Sorted in ascending order regarding wavelengths: UV-C, UV-B UV-A). The smaller is the λ, biologically the more harmful the light is.
UV-A between 320 and 400nm
UV-B between 280 and 320nm
UV-C between 100 and 280nm
Solar radiation travels through the Earth’s atmosphere before arriving at the earth’s surface. However, on this travel route, almost all UV-C radiation and 90% of UV-B radiation will be absorbed by gases such as ozone, water vapor, oxygen and carbon dioxide, together with a small part of UV-A radiation.
Therefore, the UV radiation that reaches the earth’s surface is composed of large part of UV-A radiation (95%) and a small amount of UV-B (5%).
The UV radiation that has arrived at troposphere is the engine of all photochemical processes in the lower layers of the earth’s atmosphere.
In the next Newsletter we will provide further explanation about these three types of radiation and their effects on human’s health.
You might be also interested in: